Best in class standards at scale

Five key reasons why Australia can benefit from adopting global CDISC standards
In the world of research and collecting large volumes of clinical data, sharing a ‘common language’ not only enables us to keep up with the pace of change but it improves the quality and universality of our work. As a passionate clinical research professional and Accelagen’s Head of Biostatistics, that’s how I see the Clinical Data Interchange Standards Consortium or ‘CDISC’ and the global CDISC standards.
Developed over 20 years, CDISC takes a rigorous approach to developing and advancing data standards for clinical research and beyond, and last month I was thrilled to address fellow biostatisticians at the mid-year meeting for the Australian Pharmaceutical Biostatistics Group to present on just that.
However, what soon became clear to me was that — because it is currently not necessary to follow CDISC for local work that will not be submitted to global regulatory authorities — our industry here in Australia is missing out on a host of key benefits that comes with ‘speaking the same language’ as our global affiliates.
On that note, I wanted to share five primary reasons why adopting global CDISC standards in Australia will benefit our collective industry and accelerate human health and wellness, now and into the future.
What is CDISC?
CDISC is a global not-for-profit dedicated to information system interoperability. A ‘best in class’ solution, CDISC has been designed to improve medical research and related areas of healthcare through a set of agreed upon standards.
It provides a standard structure and critical link between research, regulators and healthcare to benefit the lives of end users — in other words: a shared language.
This shared language is designed to foster the global adoption of standards that clear the way for more meaningful and efficient research and enable us to have a greater positive impact on global health.
As researchers, statisticians and clinical professionals, this set of rules not only gives us confidence and clarity that our data meets the highest standards, but it also dictates the way that our data and research looks, including: format, language, terms, and — importantly — how we frame the clinical impact garnered through our studies from conception through to regulatory submission.
Each standard is informed and shaped by the expertise of those at the forefront of research, making them not just of the highest quality, but also attuned to the practicalities and challenges that researchers, academics, pharma, statisticians, and statistical programmers might encounter when trying to implement them.
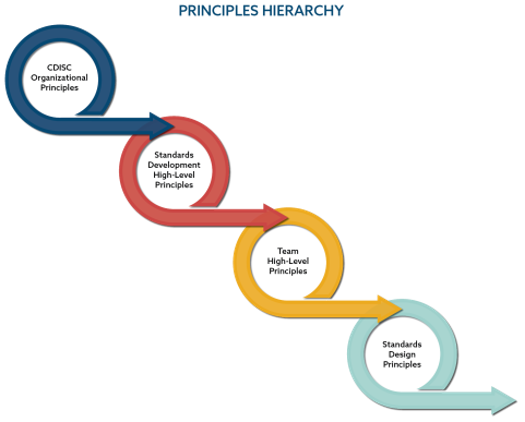
Image: CDISC Guiding Principles
Why it matters
We know that the data world is complex, and that there are many challenges — technically, logically, and practically. For that reason alone, we must look for any opportunity to ‘get on the same page,’ both literally and figuratively?
Adopting CDISC can help us achieve exactly that, no matter whether you’re a small biotech operating in Australia or a large multinational pharmaceutical company.
It is also important to note that while using CDISC standards and calculations are mandatory and somewhat ‘bread and butter’ in other parts of the world including the US, Europe, China and Japan, it is currently not required for submission to the Australian Regulatory Authority. While this can be seen as both cost and time saving measures in the short term, the reticence to adopt CDISC standards at scale could compromise local biotech’s ability to compete and collaborate on the global stage with increasing frequency in the future.
Encouraging the adoption of CDISC
Shortly after obtaining my CDISC Tabulation Certification, I finally took the plunge and began volunteering as part of the international CDISC Open Rules Engine (CORE) project in 2021.
Keen to support the programming of CDISC Conformance Rules and take advantage of any excuse to put my SDTM knowledge to use, I see it almost as my ‘duty’ to encourage the uptake and standardisation of CDISC in Australia, and to support biotech’s in our region to figure out how to best adopt these global CDISC standards in our local context.
In what is the culmination of over a decade of biostatistics and statistical programming, and (some would say) obsessive focus on CDISC, I would like to share the following five key benefits of adopting CDISC with the support of Accelagen, who share my passion for challenging the status quo and making sure that Australia takes its rightful place on the research world stage.
Benefit #1: Unlocks standardisation and accessibility
There are several examples of Australian biotech companies finding themselves facing difficulties when looking to partner or sell their data/products to big pharma, after due diligence findings highlight non-compliance to CDISC. Therefore, adopting CDISC from the beginning (or as early as possible) of a clinical program not only provides a standardised and compliant way of providing your data to a regulator, but also provides multifaceted benefits within one’s organisation, exhibiting both short and long term gains.
Implementing standards to collect, structure, and analyse data makes it easier for us as researchers and statisticians to aggregate information and utilise the abundance of data collected over the life of a clinical study and program.
CDISC helps to ensure that this data is accessible, interoperable, and reusable, unlocking the power of standardisation across contexts.
This ability to ‘speak the same language’ enables us to use research to collectively solve some of the world’s most pressing health and wellness issues, which are the real world applications for research and data that must never be far from our minds.
Benefit #2: Improves scalability and universality
Adopting global CDISC standards opens up the possibility of sharing and scaling data with clients and sponsors worldwide and is one key reason that Accelagen is both locally positioned and globally adept.
By enabling organisations to work at scale and across contexts, CDISC creates a level of portability and universality that is simply not possible without it.
An added side benefit is that it also makes internal staff recruitment easier and reduces onboarding time too — because where industry professionals are used to working the same way, we can eliminate the need to train new employees in company-specific standards.
Benefit #3: Increases efficiency and streamlining
Adopting global CDISC standards is an invaluable investment that leads to more meaningful, more efficient research for your organisation and the entire global research community.
From a CRO perspective, the adoption of CDISC standards allows the establishment of master libraries to house standardised and pre-validated CRFs, reports, statistical programs and more. This provides a framework for all subsequent studies, with efficiencies and enhanced data quality that will be seen throughout a study’s lifecycle.
Benefit #4: Reduces time to market without compromising quality
From Japan to the US, use cases show that organisations who adopt global CDISC standards produce faster, more efficient research and pave the way for more breakthroughs that amplify the power of data, in both the short- and long-term.
Critical to that path is being supported by a robust regulatory framework and having clarity in all aspects of clinical research so that you can get to market as quickly as possible, while still retaining the highest quality standards.
Benefit #5: Amplifies traceability and transparency
Traceability has always been a crucial component of scientific research, and with this in mind, CDISC has integrated data traceability right throughout their suite of standards, opening the door to greater levels of transparency and accountability for our industry to an unparalleled extent.
“Based upon reviewer experience, establishing traceability is one of the most problematic issues associated with any data conversion. If the reviewer is unable to trace study data from the data collection of subjects participating in a study to the analysis of the overall study data, then the regulatory review of a submission may be compromised.” — FDA Study Data Technical Conformance Guide
Worth the investment?
While getting up to speed on CDISC standards takes investment, in my experience, it is one that will pay off significantly, adding increasing layers of value, efficiency and quality gains over time, often in ways we might not be able to see right now.
The good news is that there is an ever-increasing range of support, education, and resources, including in-depth training and comprehensive implementation guidance, specifically designed for clinical research professionals to make adopting and using global CDISC standards as seamless as possible.
The CDISC Open-Source Alliance (COSA), in particular, further supports the adoption of CDISC through the support and promotion of open-source software projects (including CORE), which offers tools for implementing and developing CDISC standards.
To find out more, or to discuss how to better implement CDISC in your organisation, please connect with us on LinkedIn or via a meeting request.










